Nankai University team reveals new regulation mechanism for HBV’s transcription and replication
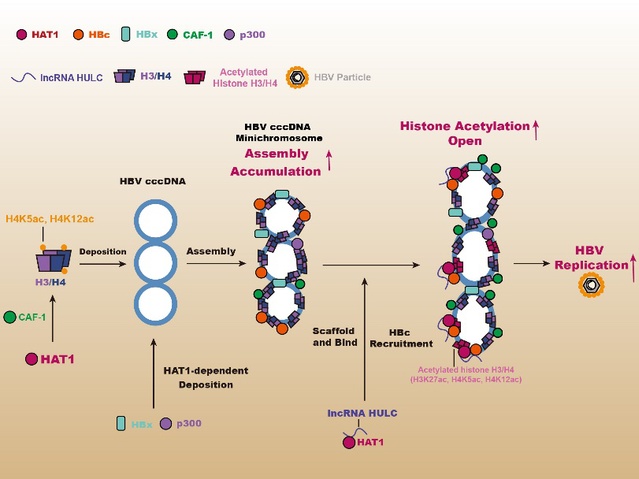
HAT1 regulates the HBV cccDNA minichromosome assembly and epigenetic modification [Photo/nankai.edu.cn]
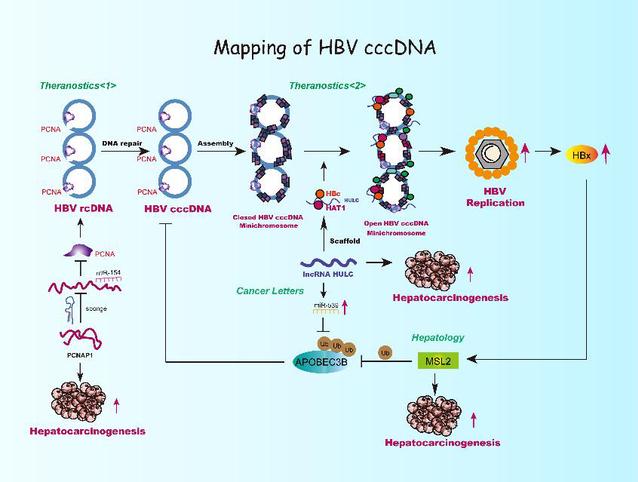
Mapping of HBV cccD [Photo/nankai.edu.cn]
The team of Professor Zhang Xiaodong of the College of Life Sciences of Nankai University, who has been engaged in the research of hepatitis B and liver cancer for a long time, has made new breakthroughs in the regulation mechanism of hepatitis B virus' transcription and replication. Recently, they published a paper in the biomedical academic journal Theranostics and revealed for the first time a new mechanism for the histone acetyltransferase HAT1 signal path promoting minichromosome HBV cccDNA (hepatitis B virus covalently closed circular DNA) assembly and epigenetic modification. It provides a new potential target for clinical clearance of HBV cccDNA and the treatment of hepatitis B.
Chronic infection with hepatitis B virus (HBV) can lead to hepatitis, cirrhosis and liver cancer. Hepatitis B virus covalently closed circular DNA (cccDNA) stands as the core of HBV's replication, and is one of the important reasons why anti-hepatitis B drugs do not completely eliminate HBV. Similar to host cell nucleosomes, HBV cccDNA forms HBV cccDNA minichromosome in the nucleus of hepatocytes by binding viral proteins such as HBx and HBc and host proteins such as histone 3/4. The structure of this minichromosome makes HBV cccDNA more stable and complete, thereby enabling the transcription and replication of HBV.
Therefore, in the process of viral host-related effects, the role of host factors in the assembly and epigenetic regulation of HBVcccDNA minichromosome and its molecular mechanisms are crucial scientific issues. At present, lack of knowledge about the molecular mechanism of HBVcccDNA minichromosome regulation makes it difficult to develop a drug that effectively eliminates HBVcccDNA. Thus, a better understanding of the process has becomes a major demand for clinical anti-hepatitis B work.
Zhang's team first established a human liver chimeric mouse model, transplanting human primary hepatocytes (PHH) into the livers of immunodeficient mice with liver injury, and then proceeding with HBV infection. This model is one of the most advanced and effective models in the field of HBV research. It can reproduce the replication process of human hepatocytes infected with HBV and maximize the clinical proximity. Interestingly, HAT1, CAF-1 and long non-coding RNA HULC are significantly expressed in HBV-infected human liver chimeric mice. The HAT1/CAF-1 signal path can promote the assembly of histone 3/4, HBx and p300 on HBVcc cDNA, and then HAT1 is involved in the acetylation of histones on the HBVcccDNA minichromosome. Molecular mechanism studies have found that HBc recruits HAT1 and anchors it to HBVcccDNA minichromosome. The long non-coding RNA HULC acts as a scaffolding to enhance the binding of HBc to HAT1, thereby forming a HAT1/HULC/HBc complex involved in the epigenetic regulation of the HBV cccDNA minichromosome. In addition, HBx can activate the transcription factor Sp1 to promote the expression of HAT1, forming a positive feedback regulation mode, which contributes to the replication of HBV.
The above studies, through the application of advanced human liver chimeric mouse models and a variety of molecular biology research methods, reveal the important role of the HAT1 signal path in the assembly and epigenetic regulation of HBVcccDNA minichromosome, and provide an experimental basis for elucidating the molecular mechanism of HBVcccDNA regulation. Understanding the molecular mechanism of HBV cccDNA microchromosome regulation through basic research is of great significance. It provides a new idea and direction for the development of anti-hepatitis B drugs and the complete elimination of HBVcccDNA.
Over recent years Zhang Xiaodong's research team has continuously published articles in internationally renowned publications such as Hepatology, Cancer Letters and Theranostics, revealing that epigenetic related factors such as male specific lethal 2 (MSL2), long non-coding RNA HULC and pseudogene PCNAP1 have the roles and molecular mechanisms to regulate HBV cccDNA minichromosome regulation, which uncovers a new mechanism for studying the replication of HBV and the development of liver cancer.
Dr. Yang Guang, a member of the research team, is the first author of the paper, and Zhang Xiaodong is its corresponding author.

Copyright ©
Tianjin Municipal Government. All rights reserved. Presented by China Daily.
京ICP备13028878号-35

